more at
Procedures for obtaining and purifying drinking water in the field.
US Army training film TF8-1174
Reupload of a previously uploaded film, in one piece instead of multiple parts, and with improved video & sound.
Public domain film from the US National Archives, slightly cropped to remove uneven edges, with the aspect ratio corrected, and one-pass brightness-contrast-color correction & mild video noise reduction applied.
The soundtrack was also processed with volume normalization, noise reduction, clipping reduction, and/or equalization (the resulting sound, though not perfect, is far less noisy than the original).
from US Army Field Manual 10-52-1
QUALITY
CHARACTERISTICS
Water quality has two major areas of concern as it relates to the daily operation of a water point. The first is the quality of the raw water source and how it affects the operation of the water purification equipment and use of treatment chemicals. The second is the quality of the product water and surveillance techniques used to guarantee its potability.
The nature of the raw water source will dictate the amount of water each purifier can produce. The total daily water requirement will indicate if additional water purification and storage equipment is needed to meet the demand. TB MED 577 lists the maximum concentration allowed for various chemicals in raw water sources. Concentrations above these limits eliminate a source as a potential water point for military operations. FM 10-52 describes in detail the effects and possible origins of physical and chemical raw water contaminates.
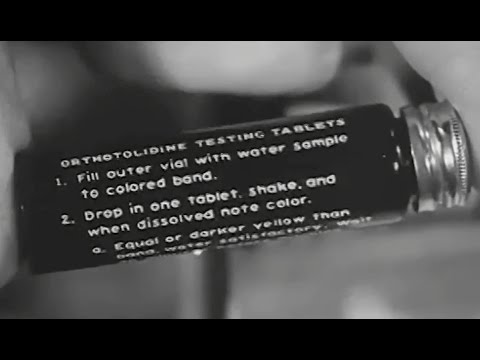
Chemical analyses and microbiological examinations of raw and treated water are required on a routine basis at water point sites. Chemical tests are necessary to ensure correct operation of the water purification equipment. Conduct chemical analyses during treatment to ensure proper chemical dosages and that the product water is potable. Conduct microbiological examinations after treatment to determine potability of the water…
RAW WATER IMPURITIES
Impurities present in raw water are in suspended, colloidal, and dissolved forms. These impurities are dissolved organic and inorganic substances, microscopic organisms, and various suspended inorganic materials. You must destabilize and bring together (coagulate) the suspended and colloidal material to form particles. Remove these particles by filtration. Colloidal material is the most difficult to remove from raw water. Processes that remove colloids from water also remove suspensions…
Once colloidal destabilization has occurred, random particle motion causes particle collision, resulting in formation of a larger particle or floc. These neutralized particles stick together forming floc masses. Bacteria particles are also neutralized (but not physically deactivated) and become entangled in the floc. As this massing continues, particle size and weight increase to a point where the larger floc can be removed by filtration…
Turbidity, pH, and color influence the coagulation of raw waters. They influence the type and amount of chemicals required. It is necessary to know which chemicals produce the most satisfactory results and to determine the exact dosage required. This can be done by test analysis or by trial runs of the equipment. The following information is essential for controlling turbidity, pH, and color in the water purification process.
Type of Turbidity
The type of turbidity plays a role in determining needs for the best pH and coagulant dosage. The following generalizations can be made:
Turbidity caused by clay concentrations requires a minimum of coagulant to provide an entangled mass of floc.
As turbidity increases, an additional coagulant dosage is generally required, although the dosage of coagulant does not increase in direct proportion to the increase in turbidity. Highly turbid waters require relatively lower coagulant dosages than low turbidity water due to the higher probability of particle collisions.
The organic matter typically adsorbed on clays in natural stream water does not significantly increase coagulant demand.
Organic colloids caused by sewage and industrial wastes are more difficult to coagulate because of extensive chemical reactions that occur between the coagulant and the colloidal organic matter.