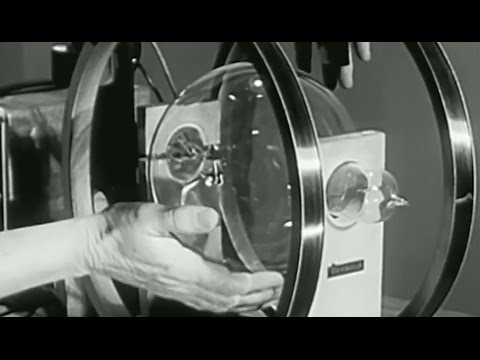
Physics & Physical Sciences playlist:
more at
“A Physical Science Study Committee LAB FILM
Professor Dorothy Montgomery
Chairman, Dept. of Physics, Hollins College”
Reupload of a previously uploaded film with improved video & sound.
Public domain film from the Library of Congress Prelinger Archives, slightly cropped to remove uneven edges, with the aspect ratio corrected, and one-pass brightness-contrast-color correction & mild video noise reduction applied.
The soundtrack was also processed with volume normalization, noise reduction, clipping reduction, and/or equalization (the resulting sound, though not perfect, is far less noisy than the original).
The electron (symbol: e−) is a subatomic particle with a negative elementary electric charge.
An electron has no known components or substructure. It is generally thought to be an elementary particle. An electron has a mass that is approximately 1/1836 that of the proton. The intrinsic angular momentum (spin) of the electron is a half-integer value in units of ħ, which means that it is a fermion. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.
Electrons, which belong to the first generation of the lepton particle family, participate in gravitational, electromagnetic and weak interactions. Like all matter, they have quantum mechanical properties of both particles and waves, so they can collide with other particles and can be diffracted like light. However, this duality is best demonstrated in experiments with electrons, due to their tiny mass. Since an electron is a fermion, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.
The concept of an indivisible quantity of electric charge was theorized to explain the chemical properties of atoms, beginning in 1838 by British natural philosopher Richard Laming; the name electron was introduced for this charge in 1894 by Irish physicist George Johnstone Stoney. The electron was identified as a particle in 1897 by J. J. Thomson and his team of British physicists.
In many physical phenomena, such as electricity, magnetism, and thermal conductivity, electrons play an essential role. An electron in motion relative to an observer generates a magnetic field, and will be deflected by external magnetic fields. When an electron is accelerated, it can absorb or radiate energy in the form of photons. Electrons, together with atomic nuclei made of protons and neutrons, make up atoms. However, electrons contribute less than 0.06% to an atom’s total mass. The attractive Coulomb force between an electron and a proton causes electrons to be bound into atoms. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding.
Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. Electrons may be destroyed through annihilation with positrons, and may be absorbed during nucleosynthesis in stars… Electrons have many applications, including in electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators…
A magnetic field is a mathematical description of the magnetic influence of electric currents and magnetic materials. The magnetic field at any given point is specified by both a direction and a magnitude (or strength); as such it is a vector field. The magnetic field is most commonly defined in terms of the Lorentz force it exerts on moving electric charges. Magnetic field can refer to two separate but closely related fields which are denoted by the symbols B and H.
Magnetic fields are produced by moving electric charges and the intrinsic magnetic moments of elementary particles associated with a fundamental quantum property, their spin. In special relativity, electric and magnetic fields are two interrelated aspects of a single object, called the electromagnetic tensor; the split of this tensor into electric and magnetic fields depends on the relative velocity of the observer and charge. In quantum physics, the electromagnetic field is quantized and electromagnetic interactions result from the exchange of photons.
…The Earth produces its own magnetic field…